Featured Solutions
Does your analytical lab analysis, testing, research and discovery take too long, cost too much and lack the data integrity, traceability and 100% assurance you need and require for your results?
ANNOUNCING MARRS 7.0
New Version
Now with “no click” automated, intelligent processing from any analytical instrument or data source through quality assurance, and primary and secondary review..all within seconds! Your lab’s data automation has never been more efficient!INTELLIGENT, SOURCE -2-SOLUTION WORKFLOW!
Compress time and eliminate errors in the lab. Knows when your instrument data is complete, grabs it and processes through 100% automated QA to primary and secondary review and more…all within seconds!
For more information and to schedule a live demo
and begin now to experience the ease of lab data automation.
PFAS
PFAS is an emerging global contamination crisis in our food and water the likes of which we’ve never before seen.How will your lab efficiently handle PFAS compliance and reporting?
Automatically generate PFAS industry compliance reports in just seconds and save hours in the lab!
- Eliminate all manual and duplicate data entry
- Save up to 8 hours per analytical sequence
- Immediate instrument integration
- Seamless universal connectivity
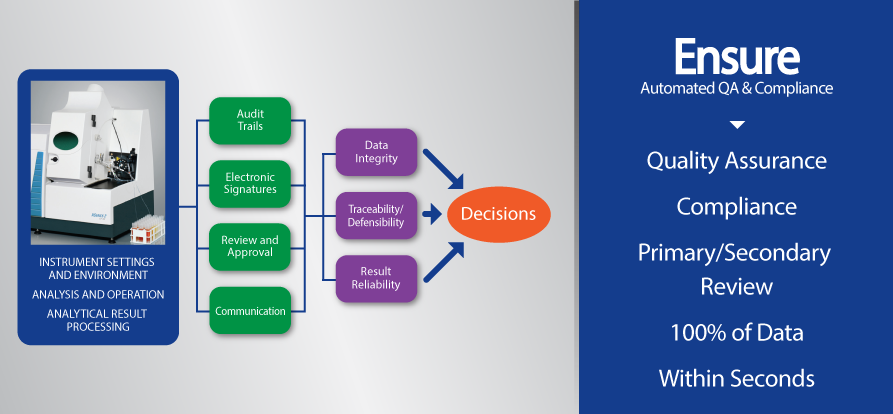
DATA INTEGRITY
Data integrity does not ensure data quality. For that, you need automated data review and quality assurance that takes just seconds!Traceable – Defensible – Reproducible – Unmask Bias
100% DATA QUALITY ASSURANCE WITHIN SECONDS
Ensure your lab’s data automatically, every time.
Traceable – Defensible – Reproducible – Unmask Bias
HARMONIZE
Brings ubiquitous scientific raw, meta, and result data to a single end point for automated quality assurance, transformation, and data sharing.SEAMLESS, UNIVERSAL CONNECTIVITY
Whether one analytical instrument, source or the entire lab, immediately integrate your instrument(s) with a downloadable link that eliminates manual data entry and seamlessly sends your data wherever it needs to go.
For more information on automated instrument & source integration or to schedule a live demo
Seamless, universal connectivity of scientific raw, meta and result data.
What’s Your Industry?
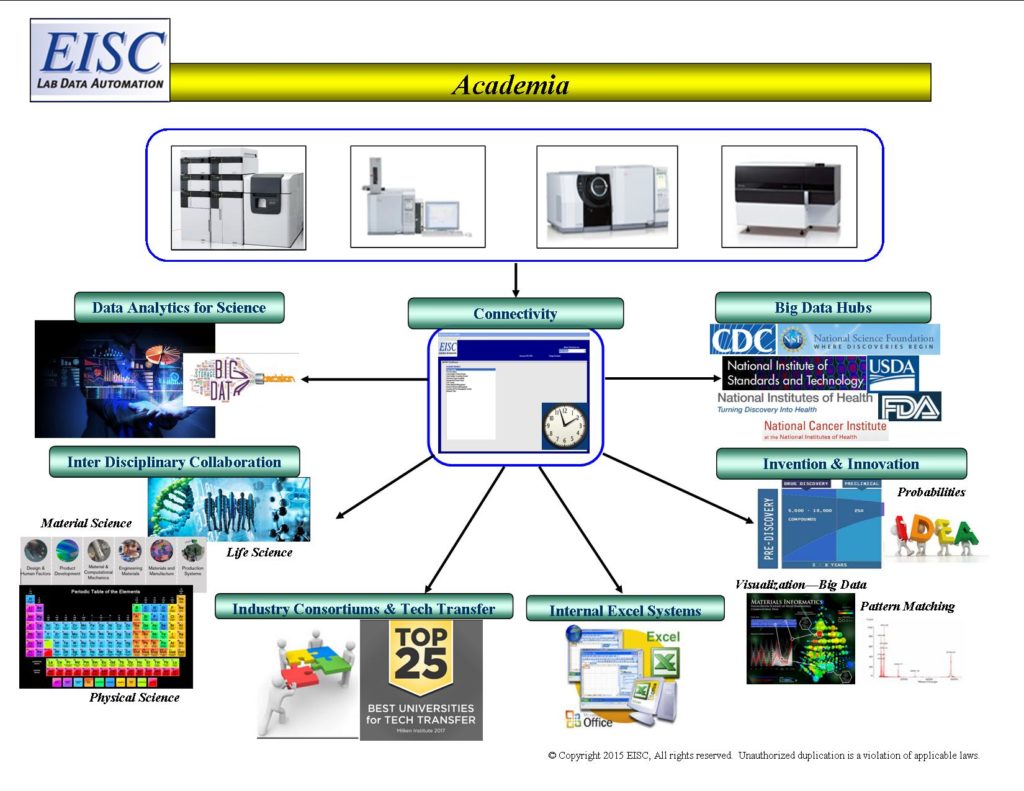
From instrument to expert decisions within minutes, immediate and traceable communication of analytical lab data.
Informatics that seamlessly access, qualify, transform and communicate laboratory analytical results.
- Electronic Reference Libraries on Instrument Data
- Internal Big Data Hubs
- External Big Data Hubs (Bi- Directional electronic integration with Federal Agencies)
- Electronic Data Deliverables to granting agencies (NSF, NIH, NIST, DOE, DOD, NASA, FDA, and more…)
- Electronic Bi-Directional connectivity of analytical results with Industry based partners, collaborators, and consortiums.
- Research Development and Technology Transfer Ecosystems
- Big Data Analytics Repositories of Scientific Data for Student Education, Statistics, Visualizations, and Discovery
- Facilitates inter-disciplinary collaboration
- Facilitates trans-disciplinary application
Request For More Information
100% quality assurance applied to transmitted data and complete traceability back to the source.
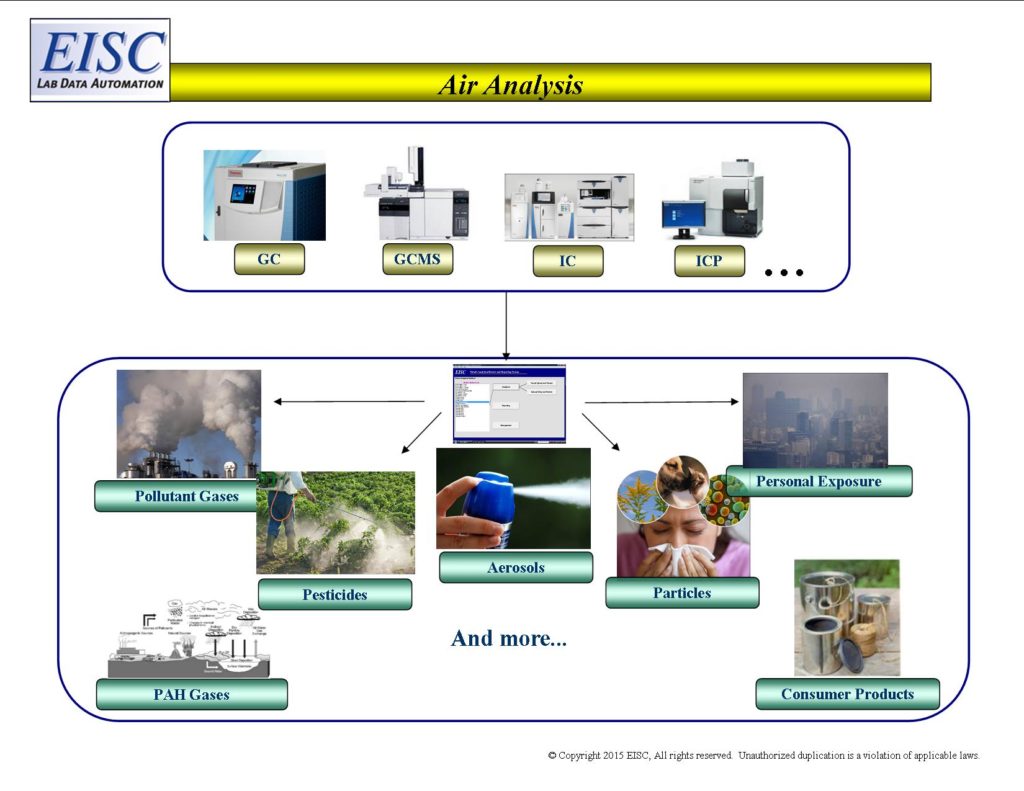
From instrument to expert decisions within minutes, immediate and traceable communication of analytical lab data.
Informatics that seamlessly access, qualify, transform and communicate laboratory analytical results.
- Personal Exposure of Air Gases
- Pollutant Gases – VOCs (Volatile Organic Compounds)
- Pollutant Gases – Pesticides
- Pollutant Gases – PAHs (Polycyclic Aromatic Hydrocarbons)
- Particles
- Filter Media – Air Filters in Residential and Industrial Sites
- Elemental Analysis
- Aerosols
- Air emissions of consumer products
- Radon
Request For More Information
Universal connectivity, 100% data quality assurance, complete traceability, one area or the entire lab.
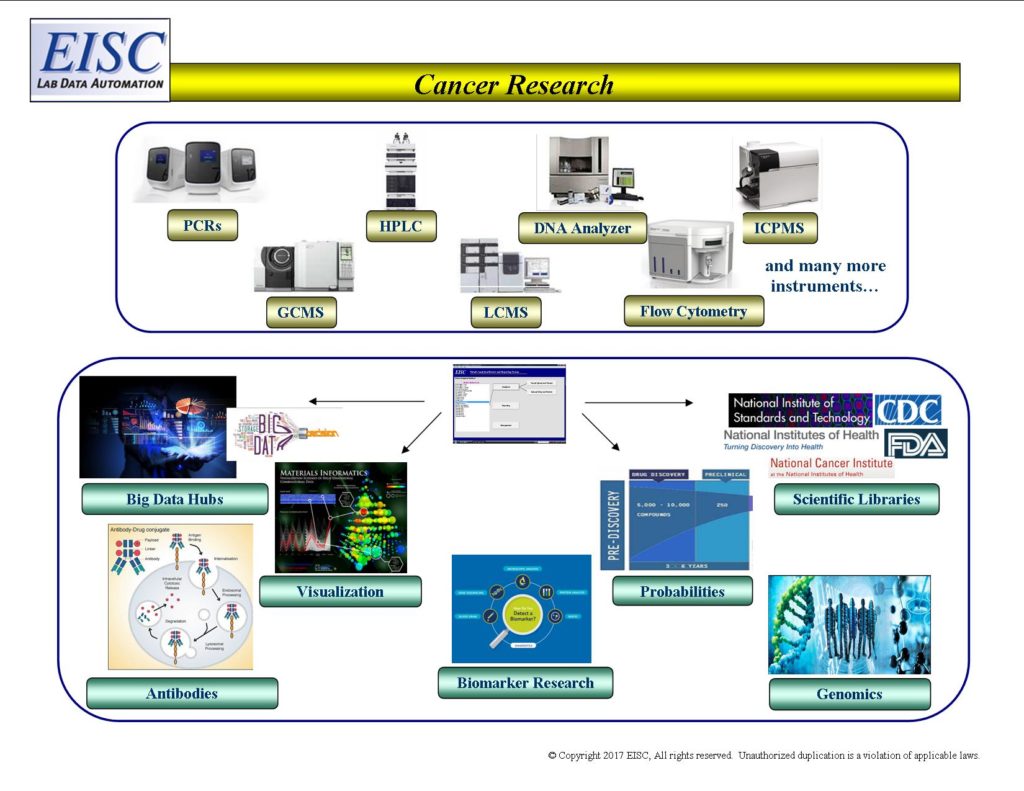
From instrument to expert decisions within minutes, immediate and traceable communication of analytical lab data.
Informatics that seamlessly access, qualify, transform and communicate laboratory analytical results.
- Internal and External Big Data Hubs
- Scientific Libraries
- Data Mining
- Data Analytics
- 3rd party Visualization tools
- 3rd party Statistical tools
- 3rd party repositories and other IMS
Request For More Information
Universal connectivity, 100% data quality assurance, complete traceability, one area or the entire lab.
As with any product, quality and safety are critical to maintaining a leading brand. Just one questionable incident has the potential to undermine the corporate stewardship that a company has invested years and even decades to build. Products in the cannabis industry are no different and MARRS 7.0 is designed to ensure your product’s quality and safety is beyond reproach. And the MARRS 7.0 automated quality assurance system brings lean six sigma discipline and methodology to the laboratory’s data process flow, electronically bridging functionality gaps that exist in within the data process flow in all labs. MARRS is modular, scalable, and compatible with any instrument type, manufacturer, model and year, LIMS or IMS and 3rd party software. It provides electronic signatures for confirmation through primary, secondary and final data review and validation for 100% data integrity, data quality, and throughput that takes just minutes.
- Compliance from instrument data access to electronic transfer or to client
- Active GMP
- GLP
- 21CFR Part 11
- ISO 17025
- Ensures
- Potency and product quality
- Flavor/aroma results and product quality
- Product safety
Express Cannabis Compliance reports automate the cannabis compliance reporting from hours, days, weeks, and even months to within just seconds for full compliance and quality assurance complete report sets. Systems are delivered via the internet.
Download PDF
Watch Video
Universal connectivity, 100% data quality assurance, complete traceability, one area or the entire lab.
From instrument to expert decisions within minutes, immediate and traceable communication of analytical lab data.
Informatics that seamlessly access, qualify, transform and communicate laboratory analytical results.
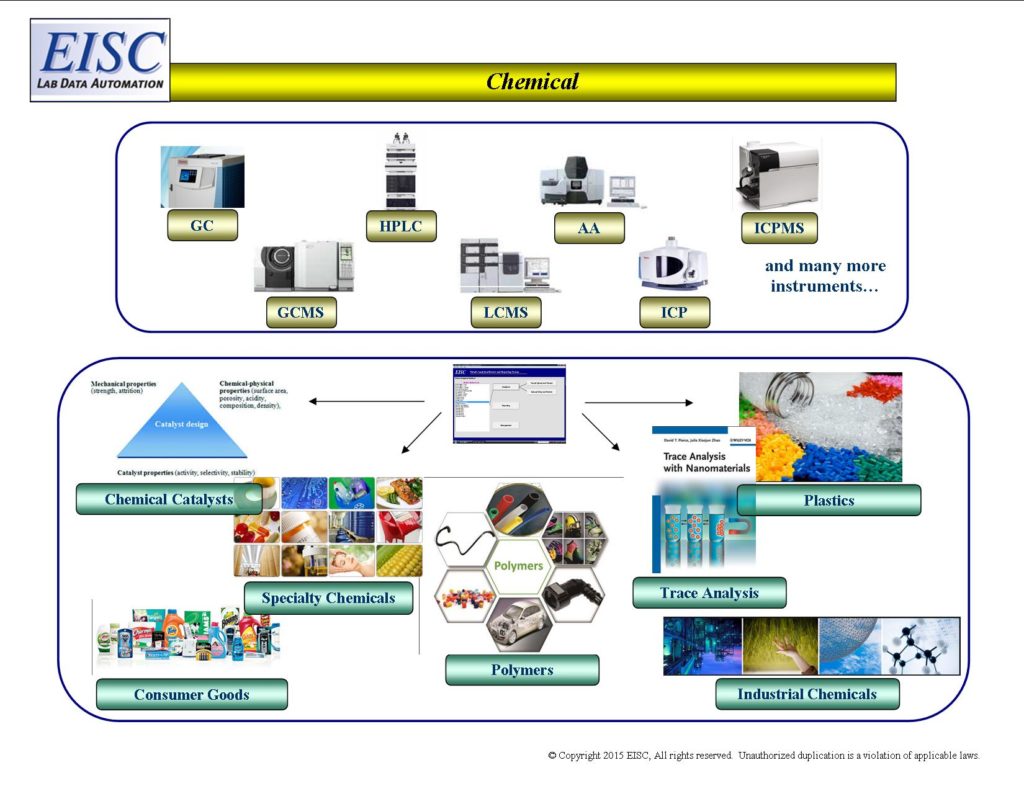
- Chemical Catalysts
- Polymers
- Plastics
- Trace Analysis
- Chemical Composition Analysis
- Contamination Detection and Analysis
- Consumer Goods
- Industrial Chemical Testing
- Specialty Chemicals
- Materials Testing
Request For More Information
Universal connectivity, 100% data quality assurance, complete traceability, one area or the entire lab.
Informatics that seamlessly access, qualify, transform and communicate laboratory analytical results.
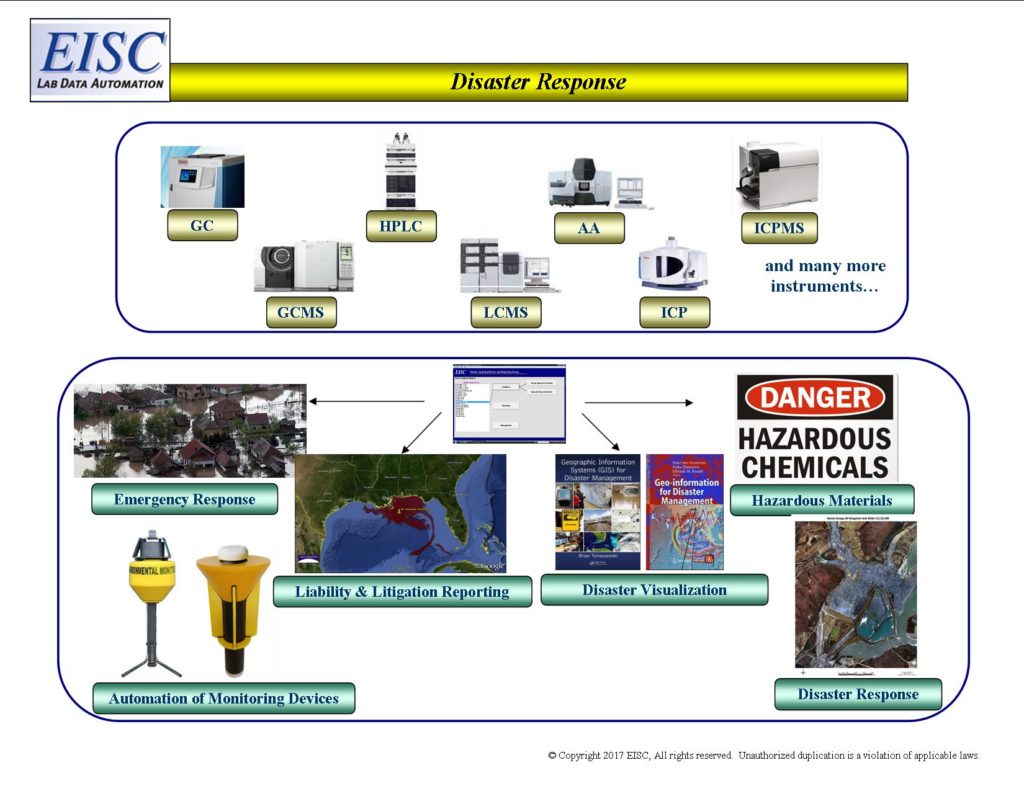
In an emergency, hazardous or disaster response, EISC systems electronically communicate analytical results from commercial laboratories to state and federal authorities within minutes.
- Analytical Automation for Emergency Response
- Analytical Automation for Hazardous Materials
- Analytical Automation for Disaster Response
- Potential Liability and Litigation Reporting of Analytical Results
- Electronic Data Deliverables for integration to 3rd Party Software
- Analytical Automation and Integration with Monitoring Devices
Request For More Information
Universal connectivity, 100% data quality assurance, complete traceability, one area or the entire lab.
From instrument to expert decisions within minutes, immediate and traceable communication of analytical lab data.
Informatics that seamlessly access, qualify, transform and communicate laboratory analytical results.
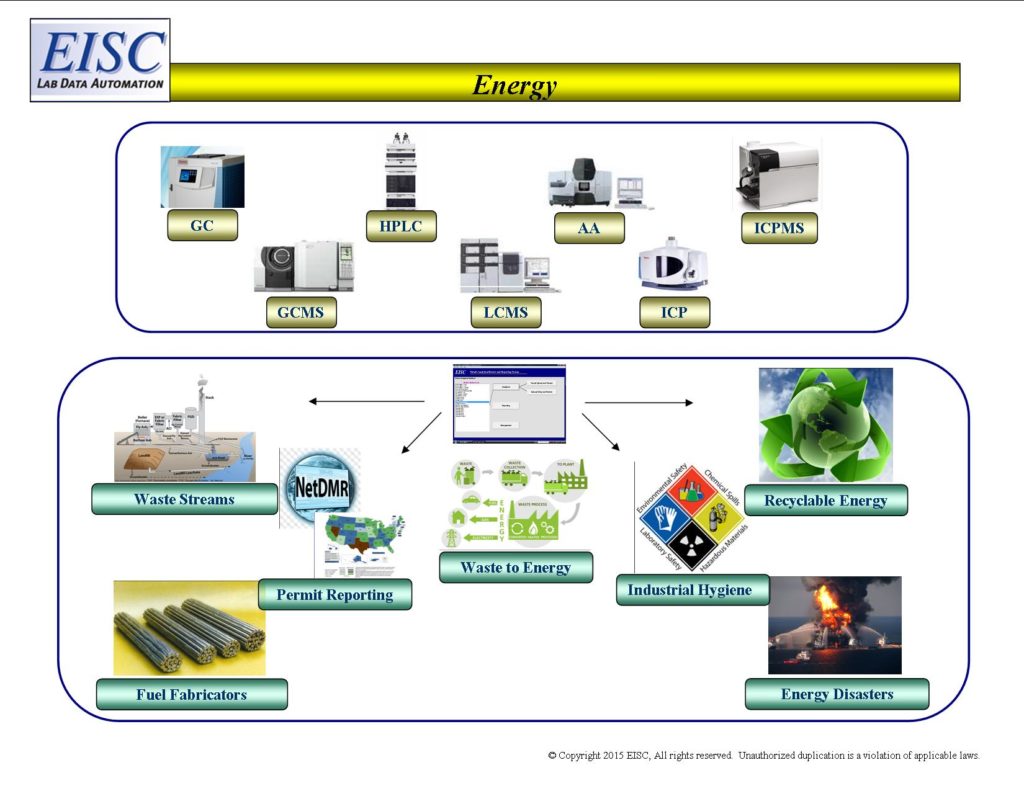
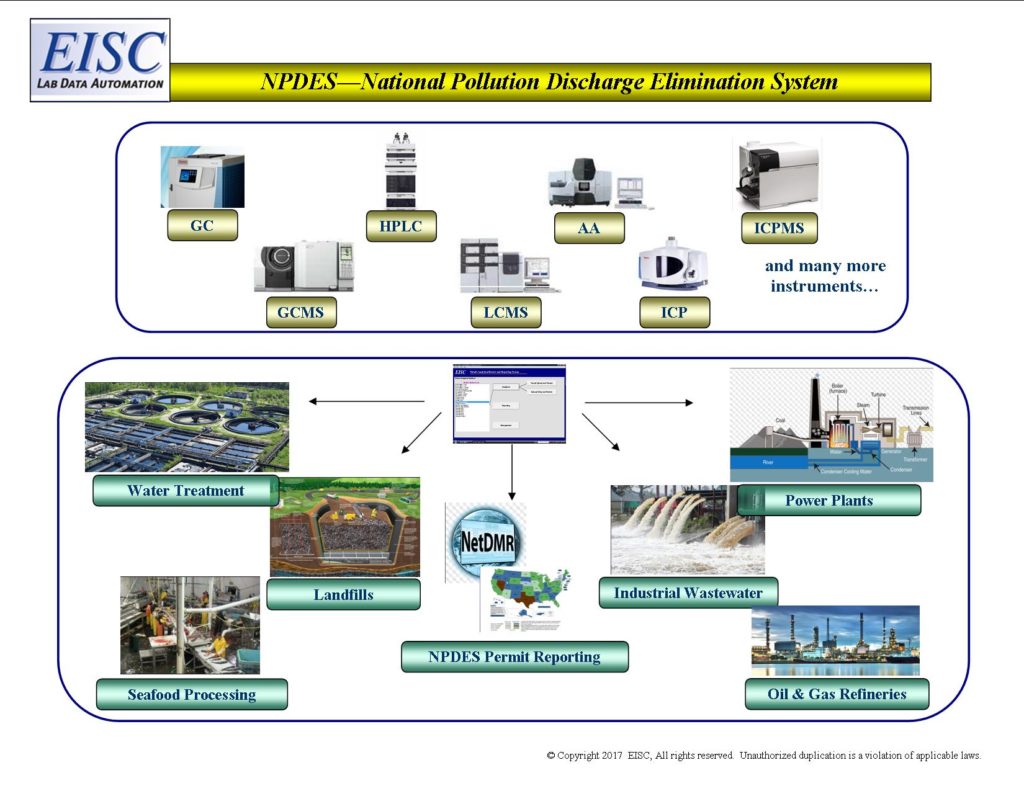
- Waste Streams of facilities (Discharge Monitoring Reports)
- NPDES (National Pollution Discharge Elimination System)
- Research and Development
- Fabricators for fuel
- Waste to Energy
- Recyclables
- Industrial Hygiene of facilities
- Disasters
- Potential Liability and Litigation Reports
Request For More Information
Universal connectivity, 100% data quality assurance, complete traceability, one area or the entire lab.
Informatics that seamlessly access, qualify, transform and communicate laboratory analytical results within minutes.
Informatics that seamlessly access, qualify, transform and communicate laboratory analytical results.
- Commercial Laboratories
- Non-Commercial Laboratories
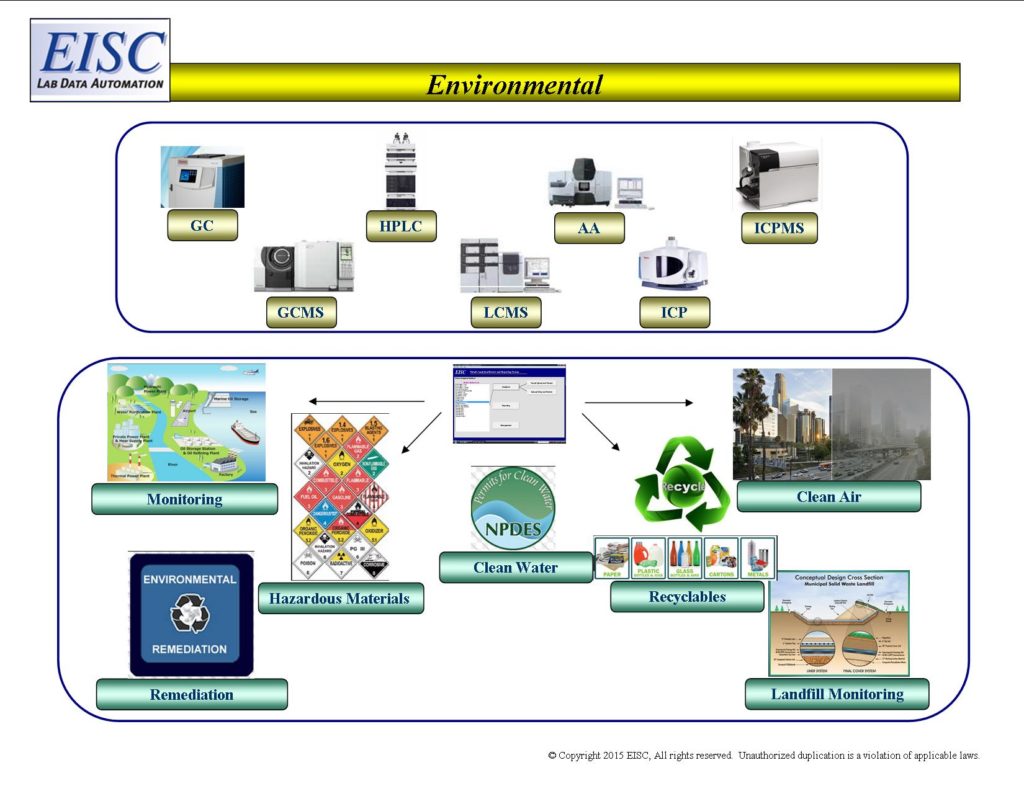
- All industries with an environmental compliance need
- Remediation
- Monitoring
- Regulatory Reporting
- Clean Water Programs
- Clear Air Programs
- Hazardous Materials
- Organic, Inorganic, Microbiology, Wet Chemistry, Radiation
- Federal Agency Laws and Programs (RCRA, CERCLA, SDWA, CWA, CAA, Superfund, Brownfields, NPDES, …)
- Analytical Result Reporting for Emergency Response and Disaster
- Analytical Result Reporting for Potential Liabilities and Litigation
- Electronic Data Deliverables for Business to Business connectivity
- Waste to Energy
- Recyclables
- Landfills
Request For More Information
Universal connectivity, 100% data quality assurance, complete traceability, one area or the entire lab.
From instrument to expert decisions within minutes, immediate and traceable communication of analytical lab data.
Informatics that seamlessly access, qualify, transform and communicate laboratory analytical results.
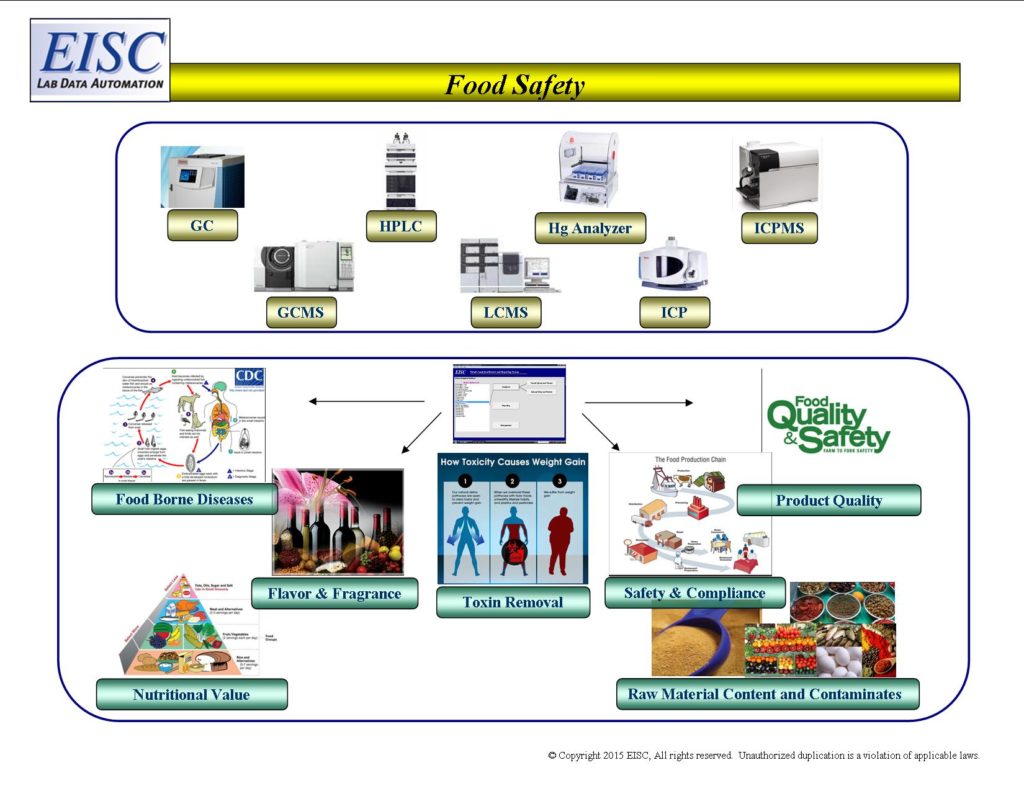
- Raw Ingredient Content and Contaminates
- Nutritional Value
- Safety and Compliance during production
- Consistency
- Toxin Removal
- Preservation
- Spoilage
- Allergens
- Food borne disease
- Processed food contaminates
- Product Quality – Flavor, Fragrance
Request For More Information
Universal connectivity, 100% data quality assurance, complete traceability, one area or the entire lab.
From instrument to expert decisions within minutes, immediate and traceable communication of analytical lab data.
Informatics that seamlessly access, qualify, transform and communicate laboratory analytical results.
Eliminate manual entry and error prone data transcription, providing the lab with a critical time savings of up to 8 hours per analytical sequence. EISC automates the communication of analytical results for
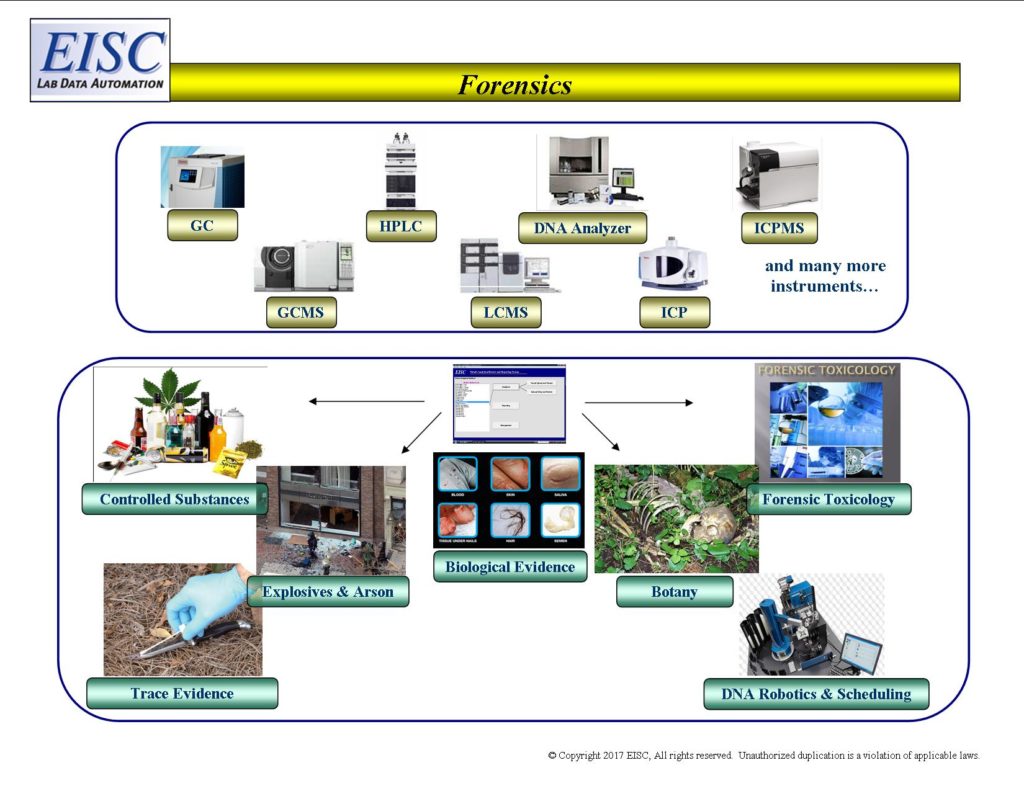
- Biological Evidence
- Trace Evidence
- Controlled Substances
- Forensic Archaeology
- Forensic Botany
- Forensic Chemistry – Illicit Drugs, Accelerants, Explosives
- DNA Instruments – Robotics and Instrument Scheduling (Non-analytical)
- Forensic Geology
- Toxicology
- Forensic Serology
Request For More Information
Universal connectivity, 100% data quality assurance, complete traceability, one area or the entire lab.
From instrument to expert decisions within minutes, immediate and traceable communication of analytical lab data.
Informatics that seamlessly access, qualify, transform and communicate laboratory analytical results.
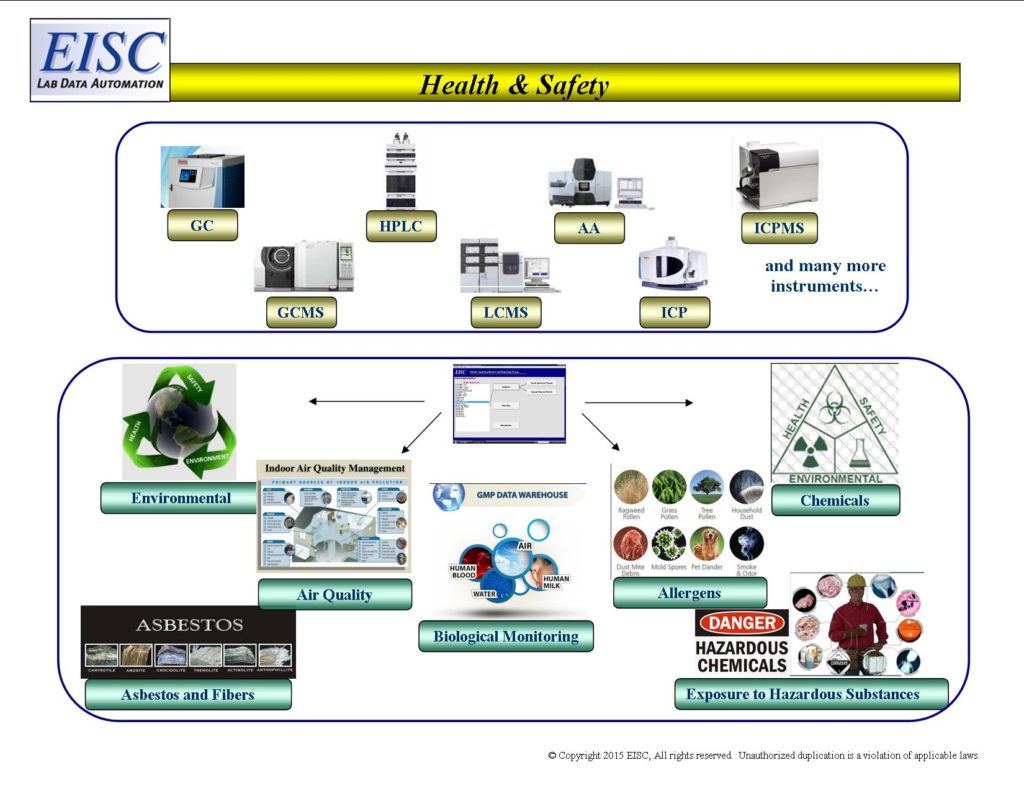
- Exposure to hazardous substances
- Biological monitoring analyses – both organic and inorganic chemicals
- Environmental analyses – analytical chemistry, real-time monitoring
- Asbestos, fibres and dusts
- Allergen monitoring – aeroallergens and enzymes
Request For More Information
Universal connectivity, 100% data quality assurance, complete traceability, one area or the entire lab.
From instrument to expert decisions within minutes, immediate and traceable communication of analytical lab data.
Informatics that seamlessly access, qualify, transform and communicate laboratory analytical results.
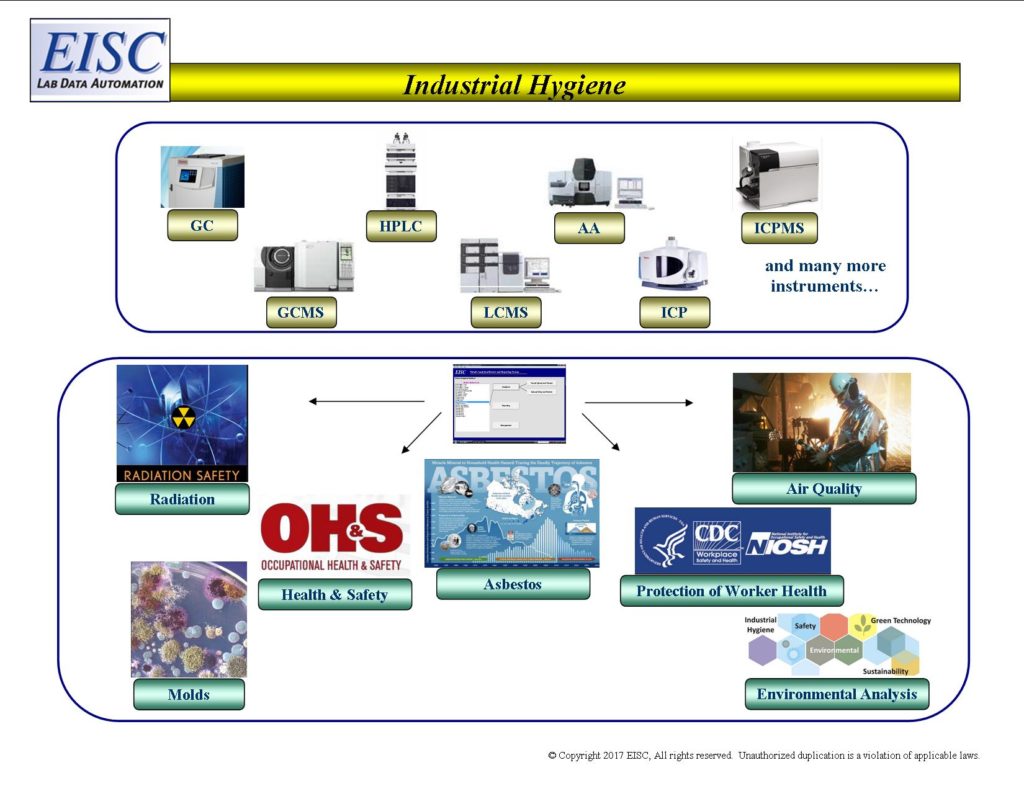
- Air Quality (see Air)
- Asbestos
- Radiation
- Molds
- Health and Safety Analysis
- Environmental Analysis
- Protection of Worker Health
Request For More Information
Universal connectivity, 100% data quality assurance, complete traceability, one area or the entire lab.
From instrument to expert decisions within minutes, immediate and traceable communication of analytical lab data.
Informatics that seamlessly access, qualify, transform and communicate laboratory analytical results.
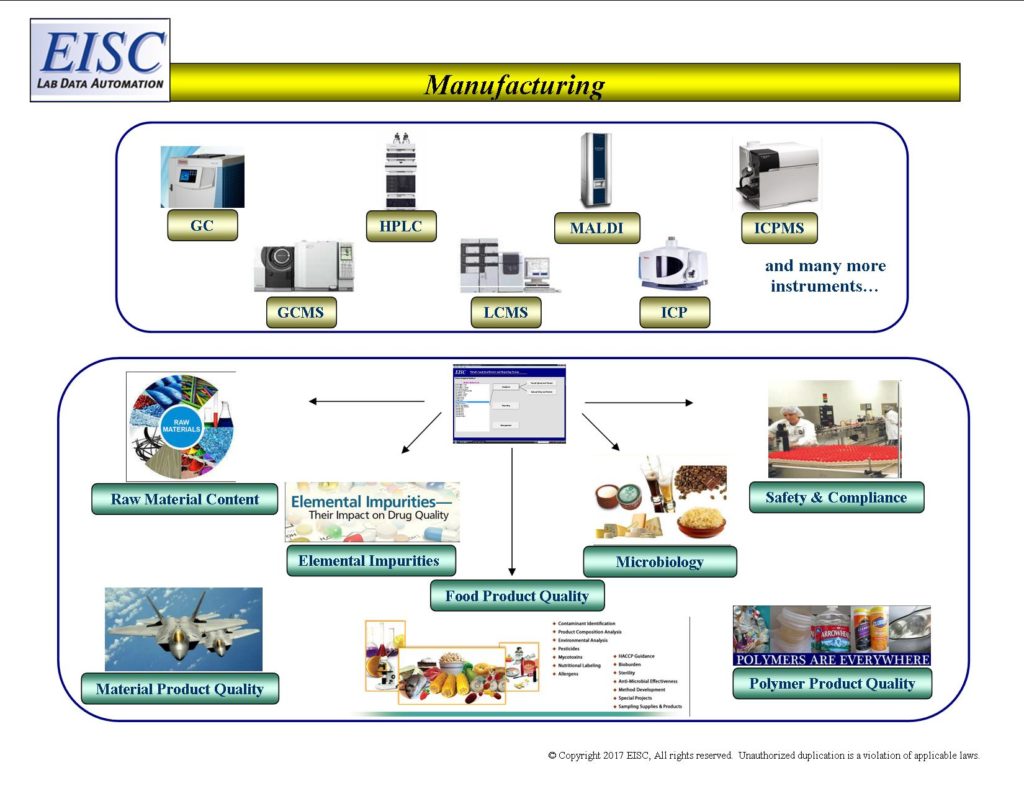
- Raw Material Content
- Safety and Compliance during production
- Interferant Reporting
- Analytical based fabrication reports
- Recyclables
- Product Quality
- Product compliance testing
- Product emissions testing
Request For More Information
Universal connectivity, 100% data quality assurance, complete traceability, one area or the entire lab.
From instrument to expert decisions within minutes, immediate and traceable communication of analytical lab data.
Informatics that seamlessly access, qualify, transform and communicate laboratory analytical results.
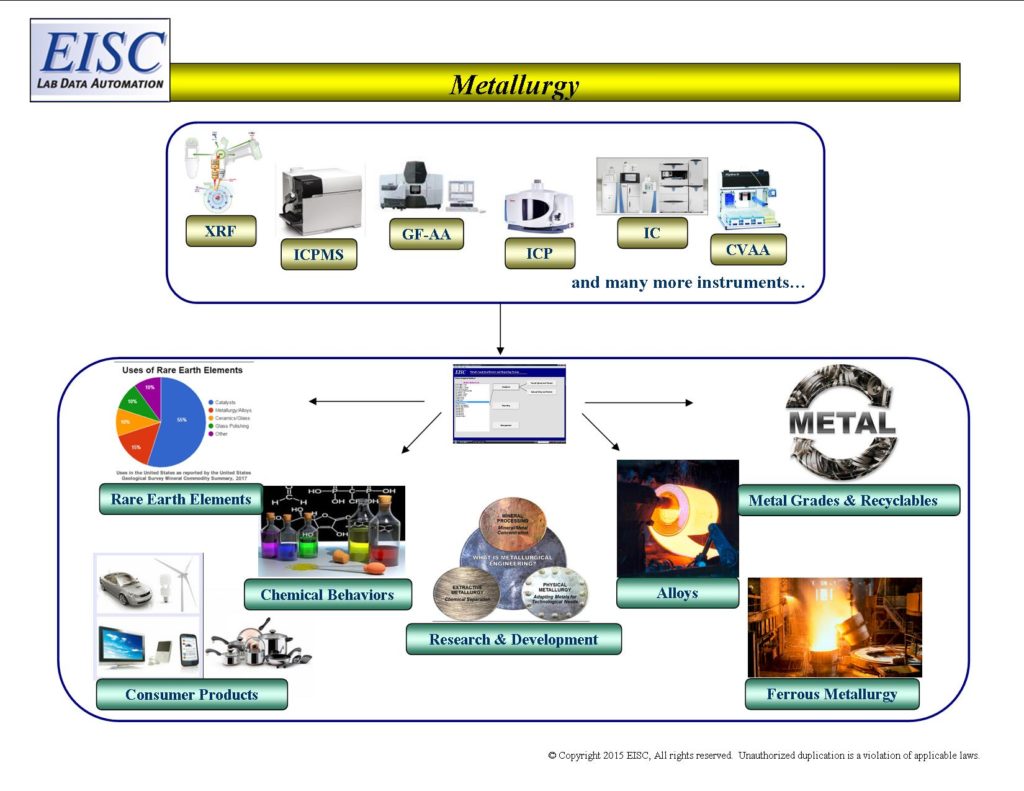
- Research and Development
- Chemical behaviors
- Alloy content
- Metal grade
- Metals components for consumer and manufacturer products
- Product Quality
- Product compliance testing
- Rare Earth elements
- Ferrous metallurgy
Request For More Information
Universal connectivity, 100% data quality assurance, complete traceability, one area or the entire lab.
From instrument to expert decisions within minutes, immediate and traceable communication of analytical lab data.
Informatics that seamlessly access, qualify, transform and communicate laboratory analytical results.
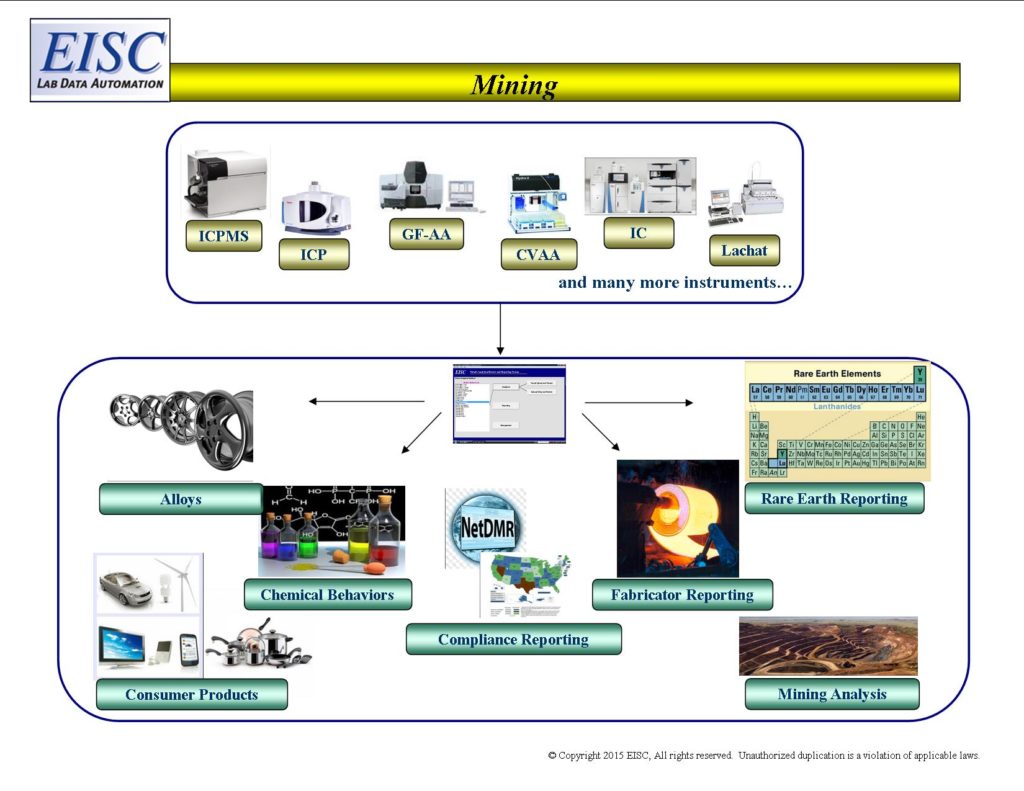
- Integration of Analytical Results with 3rd Party Software products for business modeling, efficiency, productivity, and throughput.
- Elemental Recovery, Content, and Interferent Reporting
- Reporting to Fabricators
- Alloy content
- Environmental Compliance
- Metals components for consumer and manufacturer products
- Rare Earth elements
- Health & Safety Group/Industrial Hygiene (OSHA)
- Environmental Compliance Group/Federal & Local Environmental Agencies (State, EPA)
- Manufacturing facility for fabrication. Determines percentages of metals and interfering elements
- Companies making the products for the mining operation. Determines percentages of metals in metal alloy sheets.
Request For More Information
Analytical – Environmental Compliance – Water Treatment – Fabrication – Manufacturing
From instrument to expert decisions within minutes, immediate and traceable communication of analytical lab data.
Informatics that seamlessly access, qualify, transform and communicate laboratory analytical results
- Animal Feeding Operations (AFOs)
- Aquaculture
- Biosolids
- Bulk Fuel Storage
- Contaminated Groundwater Treatment
- Domestic Wastewater Treatment Facilities
- Electric Generating Facilities
- Industrial Wastewater
- Landfills, Land Application Sites, and Open Dumps
- Mining and Quarries
- Municipal Separate Storm Sewer System (MS4)
- Municipal Wastewater
- Oil & Gas Extraction
- Pesticide Application
- Seafood Processing
- Sludge
- Stormwater
- Temporary Discharges
- Vessels, Marinas, and Boatyards
- Water Treatment Facility (potable & non-potable)
- Watershed Permits
- Others
Request For More Information
Universal connectivity, 100% data quality assurance, complete traceability, one area or the entire lab.
From instrument to expert decisions within minutes, immediate and traceable communication of analytical lab data.
Informatics that seamlessly access, qualify, transform and communicate laboratory analytical results.
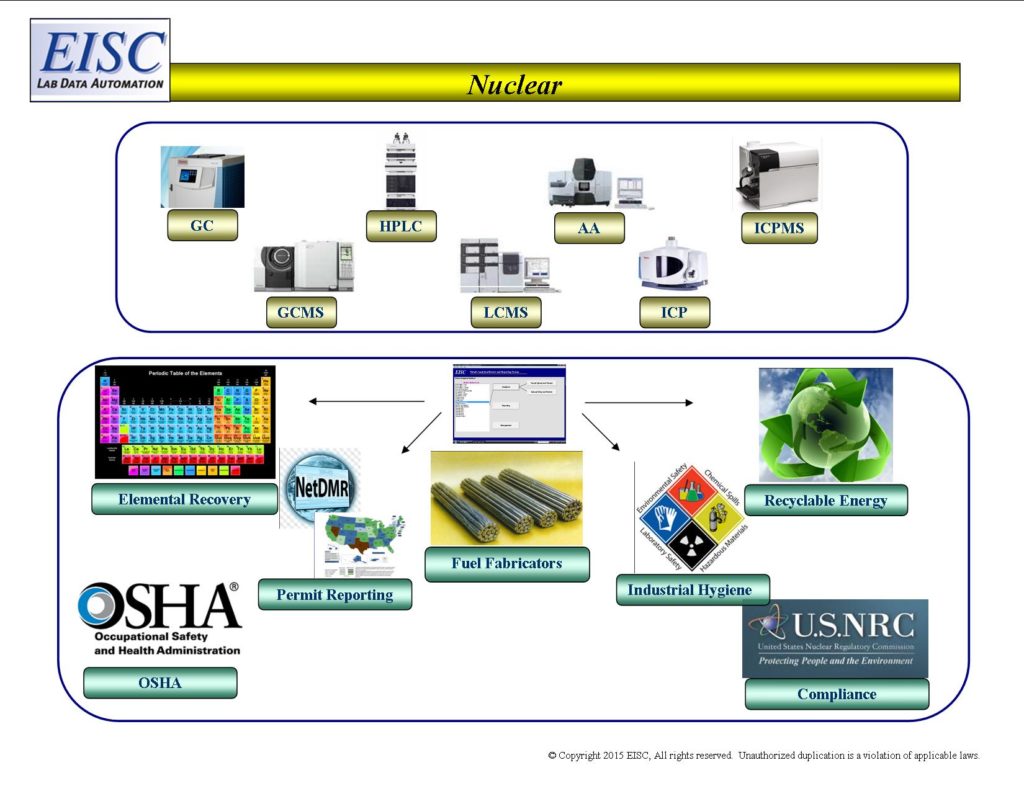
- Analytical Result Reporting for Fabrication of Nuclear Energy
- Health and Safety of Employees
- Industrial Hygiene
- Environmental Compliance
- Nuclear Regulatory Agency Compliance
- Analytical Result Reporting for Nuclear Enrichment
- Integration of Analytical Results with 3rd Party Software products for business modeling, efficiency, productivity, and throughput.
- Elemental Recovery, Content, and Interferant Reporting
Request For More Information
Universal connectivity, 100% data quality assurance, complete traceability, one area or the entire lab.
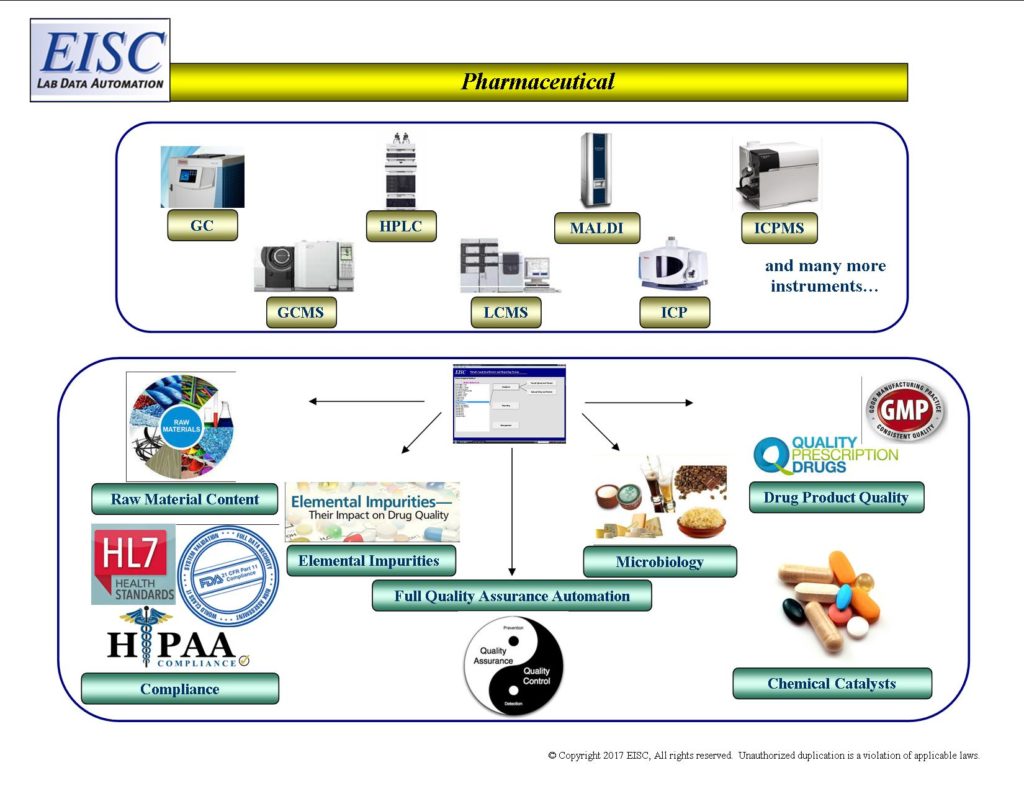
Its widely known that getting a drug or cure to market can take 8-10 yrs and cost $1 billion+
When applied within pharma, EISC systems compress the time normally needed in early research due to just a fraction of the time due to the automation of applicable analytical lab data processes. Additionally, the automated quality assurance ensure that one not only gets to discovery faster, but to the right discovery faster with 100% traceability that tracks every decision and judgement applied to the science throughout the data’s journey. This ensures that any bias present within the data/study is visible for those associated with the science, including investors. Fail fast/Fail Cheap.
Informatics that seamlessly access, qualify, transform and communicate laboratory analytical results for
- 21 CFR Part 11 Compliance
- HIPAA Privacy Compliance
- HL7 Electronic Connectivity to Instruments
- Full Quality Assurance Automation
- Internal/External Big Data Hubs Bi-Directional Connectivity
- Internal/External Reference Library Bi-Directional Connectivity
- Instrument Interfacing and Integration
- Integration of Analytical Results with 3rd Party Software products for statistics, modeling, algorithms, probabilities, visualization …
Request For More Information
With Pharmaceuticals, EISC systems communicate analytical results into Intranet Big data hubs. EISC systems can then interact with internet available International Reference Libraries.
Same instruments, similar analytical methods, different uses of the data presented in the way a specific expert needs the analytical data communicated and reported.
From instrument to expert decisions within minutes, immediate and traceable communication of analytical lab data.
Informatics that seamlessly access, qualify, transform and communicate laboratory analytical results.
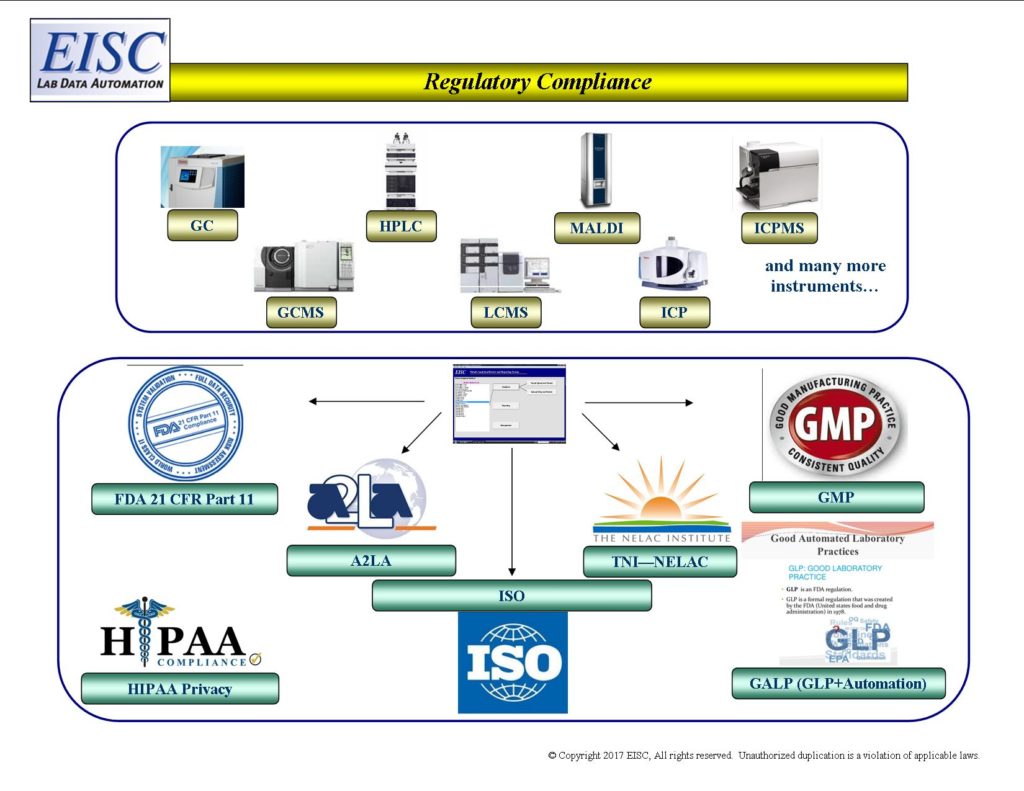
EISC automates the compliance for
- FDA 21 CFR Part 11
- HIPPA Privacy
- HACCP
- CDC
- DOE
- DOD
- DOJ
- EPA
- ISO
- GMP
- GALP
- NELAP
- USDA
- Nuclear Regulatory Commission
Request For More Information
Universal connectivity, 100% data quality assurance, complete traceability, one area or the entire lab.
From instrument to expert decisions within minutes, immediate and traceable communication of analytical lab data.
Informatics that seamlessly access, qualify, transform and communicate laboratory analytical results.
- Internal and External Big Data Hubs
- Scientific Libraries
- Data Mining
- Data Analytics
- 3rd party Visualization tools
- 3rd party Statistical tools
- 3rd party repositories and other IMS
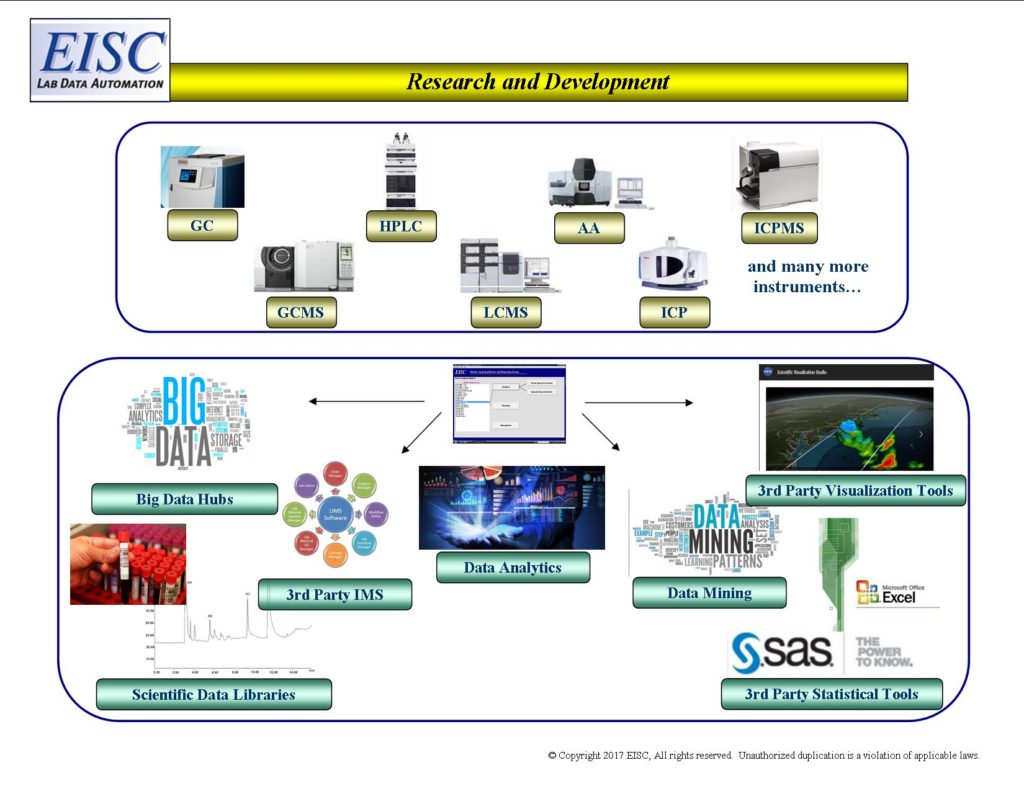
Request For More Information
Universal connectivity, 100% data quality assurance, complete traceability, one area or the entire lab.
From instrument to expert decisions within minutes, immediate and traceable communication of analytical lab data.
Informatics that seamlessly access, qualify, transform and communicate laboratory analytical results.
- Landfills
- Recyclables
- Hazardous Waste
- Nuclear Waste
- CERCLA (Superfund) – Comprehensive Environmental Response, Compensation and Liability Act
- RCRA (Management of Non-Hazardous and Hazardous Solid Waste) Resource Conservation and Recovery Act
- Brownfields – Clean up and Re-investment in contaminated properties for re-use
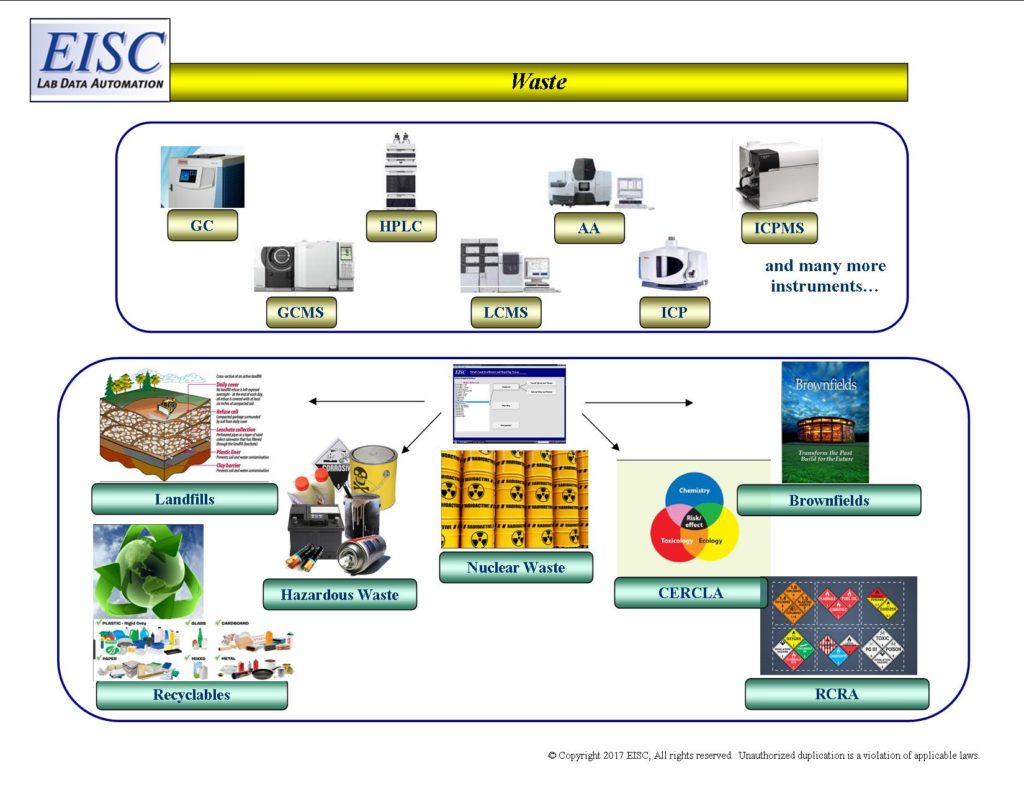
Request For More Information
Universal connectivity, 100% data quality assurance, complete traceability, one area or the entire lab.
From instrument to expert decisions within minutes, immediate and traceable communication of analytical lab data.
Informatics that seamlessly access, qualify, transform and communicate laboratory analytical results.
- Environmental Compliance Group/Federal & Local Environmental Agencies (state,
- Water Treatment Facility: Cleanup issues that need to be addressed during the Water Treatment process
- Disposal of solid waste to landfill: Sludge results need to meet compliance requirements for landfill disposal
- Renewable Energy: converting a Sludge bi-product into renewable energy.
- Big data hubs for internal or same instruments, similar analytical methods, different uses of the data presented in the way a specific expert needs the analytical data communicated and reported.
- Analytical Automation for Drinking Water Treatment Plants
- Analytical Automation for Waste Water Treatment Plants
- Analytical Automation for Waste Streams
- National Pollution Discharge Elimination System (NPDES)
- Safe Drinking Water Act (SDWA)
- Clean Water Act (CWA)
- Priority Pollutants
- Persistent organic pollutants (POPs)
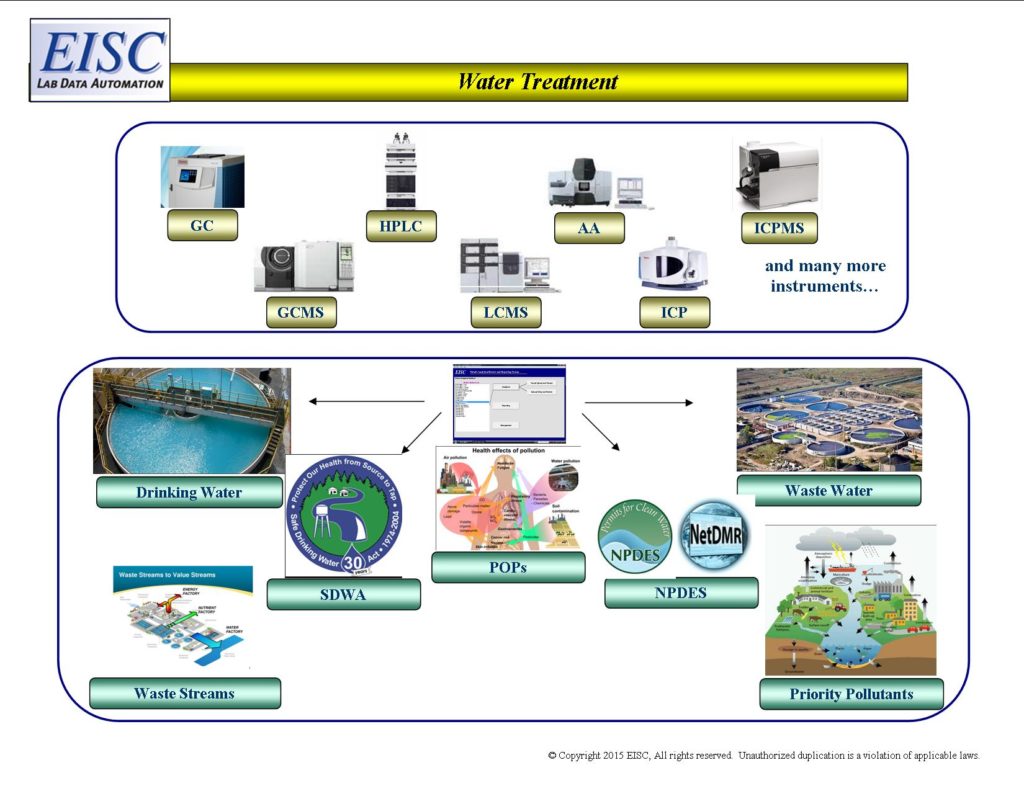
Request For More Information
Clean water – Landfills – renewable energy – environmental compliance – transfer to NetDMR – Published to the Web
What’s Your Greatest Obstacle?
Whether 21 CFR Part 11 or another electronic regulatory standard, make any analytical instrument in your lab immediately compliant and ensure all of your lab’s data is traceable and defensible.
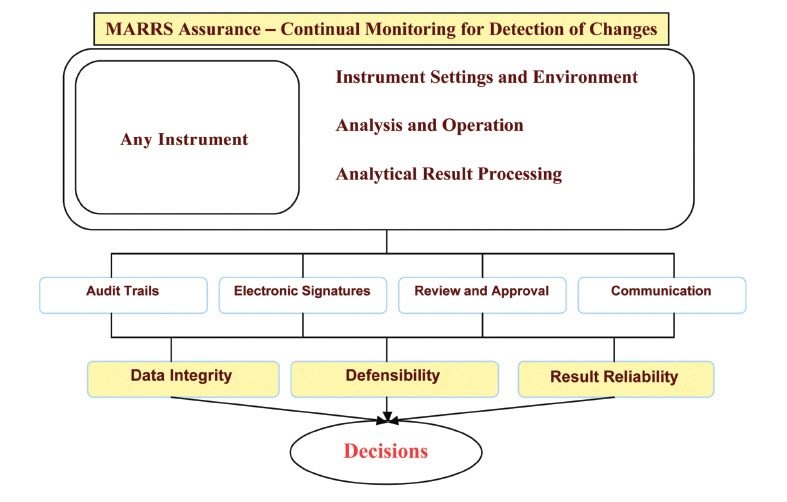
MARRS Assurance automatically integrates with any analytical instrument and throughout the entire lab with capabilities that allow the lab to personalize the software in order to meet their lab’s dynamic security needs. Achieve immediate compliance with electronic regulatory standards including, but not limited to:
- 21 CFR Part 11
- Good Manufacturing Practices (GMP)
- Good Automated Laboratory Practices (GALP)
- …CDC, DOE, DOD, EPA, HACCP, ICO, NELAP, USDA, Nuclear Regulatory Commission in data handling
- Seamlessly integrates within current lab processes with no decrease in production
- Automates and streamlines QA review
- Communicates analytical issues throughout the laboratory
- Ensures data quality and integrity
- Continual monitoring of instrument files, folders, tables, electronic signatures, versioning, audit trails
- Continual monitoring of electronic signatures, electronic versioning, and audit trails
- Work flow/Process flow definition
- Review checklists
- Instant messaging
- Vendor & Industry neutral
- Delivered via the internet
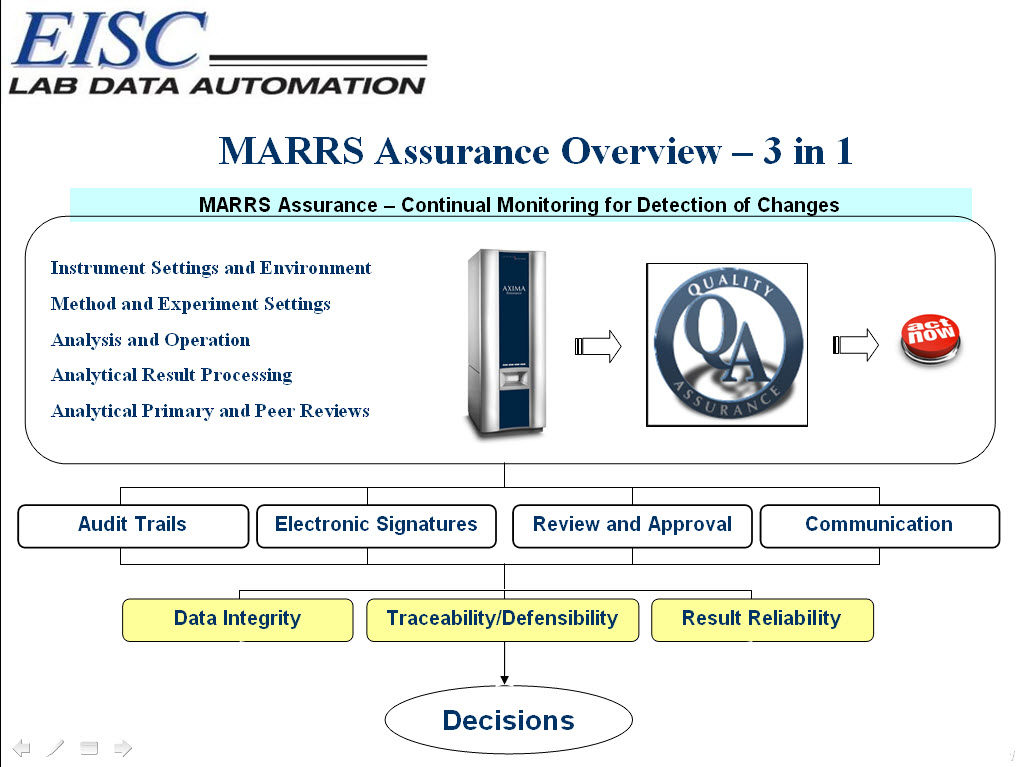
Request For More Information
100% quality assurance applied to transmitted data and complete traceability back to the source.
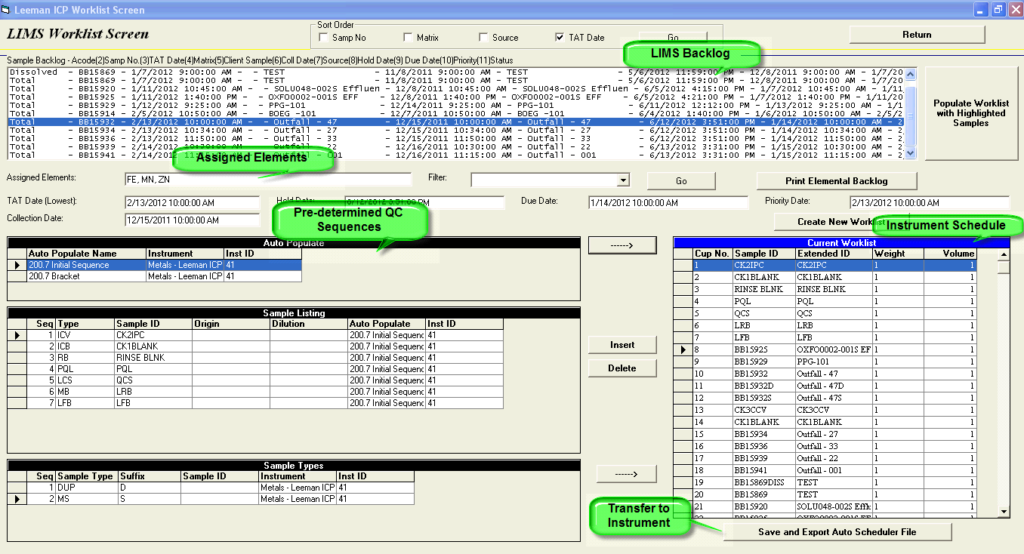
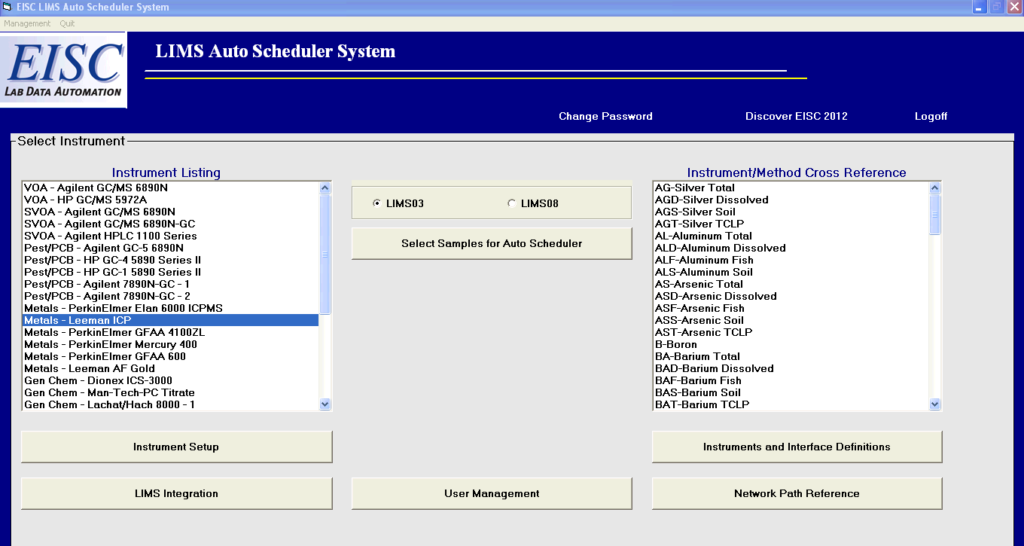
Automating the LIMS to Instrument Sample Schedule
EISC’s AutoScheduler provides an immediate solution to an unrecognized opportunity for saving hours within the lab: Automating the population of instrument sample schedules directly from a laboratory information management system’s (LIMS) backlog. EISC’s AutoScheduler removes the manual entry of Sample ID and sample information into the instrument software, and immediately automates the transfer of a laboratory’s LIMS backlog and batches to the instrument software. This vendor and industry neutral software integrates any lab instrumentation with the capability to receive an electronic transfer.
Automate your LIMS to Autoscheduler …and save hours in lab production!
Remove the manual entry of sample ID and sample information by automating your LIMS backlog and batches to the instrument software.
BENEFITS:
- Save up to 2 hrs. per analyst/per instrument/per day*
- Eliminates manual entry of sample ID/info.
- Immediate/dramatic production increase
- Vendor/Industry neutral
- Self implement or utilize our support team
- Ensures data quality & Integrity
Click at right to view a short demo of EISC’s AutoScheduler at work. Then, contact us to find out how your lab can begin to experience the benefits of automated sample scheduling right away!
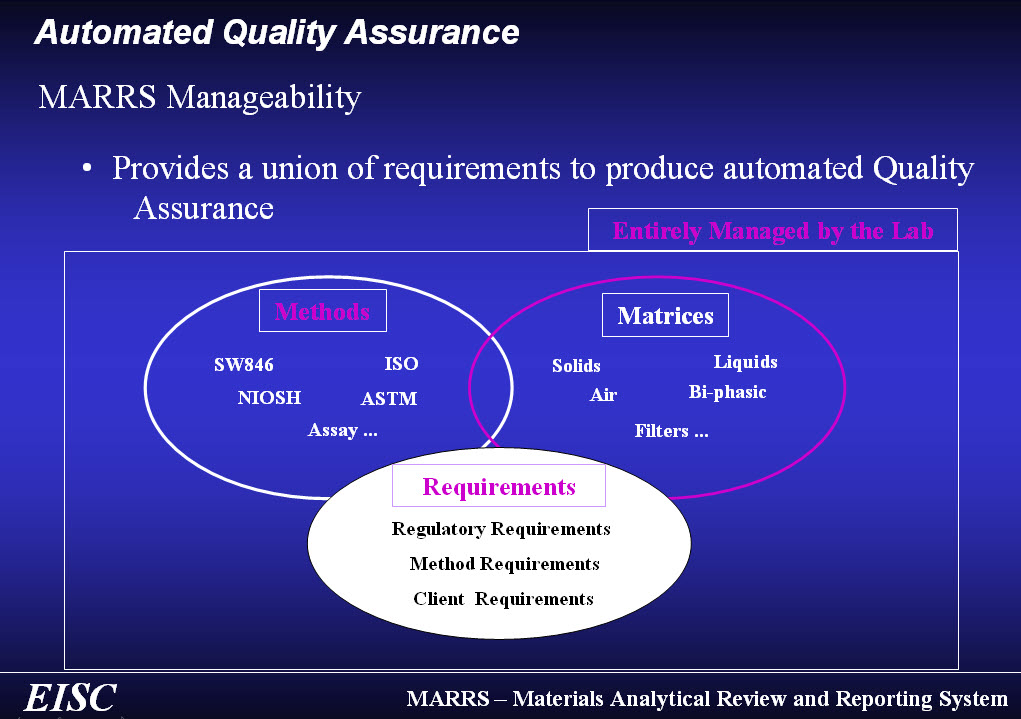
Still entrusting your lab’s quality assurance to manual data entry and calculation? Your lab’s analytical QAPP…are you really sure it’s being followed by everyone in the lab?
Quality assurance is non-negotiable in the lab. So, whether standalone, direct from any instrument, through our systems, or integrated with your LIMS or ELN, our automated quality assurance provides immediate lab data quality assurance across the lab…within seconds!
- Saves countless hours in the lab…QA/data review within seconds
- 100% of data
- Both primary and secondary review
- Direct from any analytical instrument(s)
- Ensures data quality & integrity
- Makes data bias visible, brings it to the forefront
- Complete data traceability & defensibility
- Standardizes lab’s QAPP
- Seamlessly combines method, regulatory, and client requirements across instrument, prep and sample QC
- Vendor & Industry neutral
- Stands alone or fully integrates with current and future IMS
- Bi-directional IMS connection
- Modular and scalable for additional lab data automation features and functionality
- Complies with industry standards (HIPPA, ISO, 21CFR Par 11, etc…)
- Delivered via internet
Request For More Information
100% quality assurance applied to transmitted data and complete traceability back to the source.
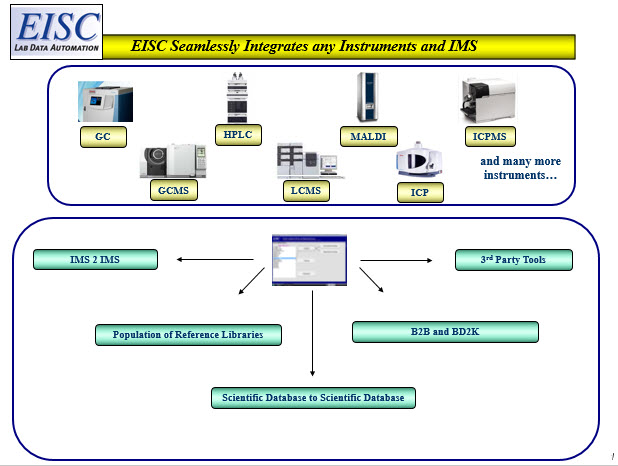
Integrate and harmonize any analytical instrument(s) in your lab today!
Whether it’s one instrument or the entire lab, EISC’s patented automated instrument integration technology lets you integrate and harmonize any and all instruments to wherever your raw, meta or final results need to go…automatically. Our systems bring disparate instrument data sources to a single point of process for scientific review and automated QA/QC that accelerates science and helps you get to discovery faster.
- Significant time savings in the lab…up to 8 hrs. per analytical sequence
- Eliminates manual entry of data, minimizing human error
- 100% data traceability
- Ensures data integrity
- Any analytical instrument, make model, year, software
- Vendor/Industry neutral…any IMS (LIMS, Excel Spreadsheets, Central Repositories, Reports)
- One instrument or the entire lab
- Harmonizes different instrument manufacturers and software into one seamless transfer
- Accelerates instrument implementation into lab production process
- Immediate ROI (Return on Instrument Investment)
- Any sample/QC type results
- Modular and scalable for QA automation/standardization
- Modular and scalable for additional lab data automation features and functionality
- No change to your lab’s current data process or workflow
- Significant increase in production
- Speed to discovery
- Speed to sound decisions
Request For More Information
100% quality assurance applied to transmitted data and complete traceability back to the source.
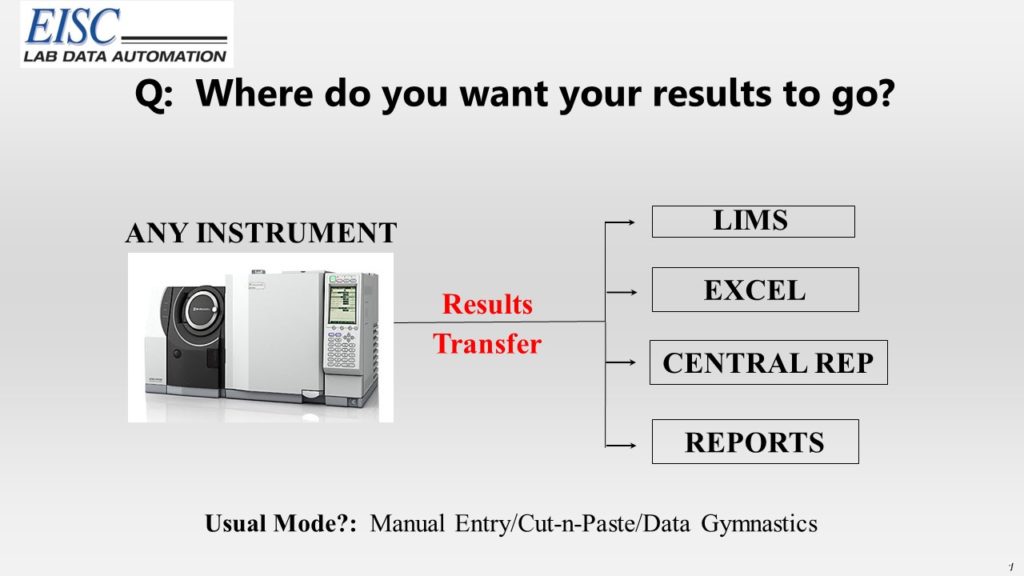
EISC systems immediately automate the transfer of analytical results from any analytical instrument to wherever those results need to go!
- Any LIMS or IMS
- Brand or internally developed
- Bidirectional connection
- Significant time savings in the lab…up to 8 hours per analytical sequence!
- Eliminates manual and duplicate data entry
- No downtime in the lab
- Integration of instruments to management systems
- Vendor & industry neutral
- Delivered via the internet

Request For More Information
100% quality assurance applied to transmitted data and complete traceability back to the source.
Automatically generate any client specific or regulatory reports within seconds!
Whether direct from the instrument, through our systems, or through your lab’s LIMS/IMS, EISC’s lab data automation systems do all of the work required for generating any reports for you so you no longer have to perform the data gymnastics you’ve been doing in the past in order to get your work done.
- Generate any client specific or regulatory reports
- Applicable to any analytical industry
- Full quality assurance reports
- Regulatory report modules for defensibility
- Regulatory report modules for potential litigation or liability results
- Delivered via the internet

Request For More Information
100% quality assurance applied to transmitted data and complete traceability back to the source.
Automatically generate any client specific or regulatory electronic data transfers (EDT’s) and facilitate business to business scientific communication within seconds!
Whether direct from the instrument, through our systems, or through your lab’s LIMS/IMS, EISC’s lab data automation systems do all of the work required for generating any EDT’s for you so you no longer have to perform the data gymnastics you’ve been doing in the past in order to achieve our analytical scientific goals.
- Allows separate entities to act as one
- Builds a business relationship based on seamless connectivity and need
- Establishes data quality and integrity between separate entities
- Facilitates scientific collaboration
- Flexible to meet any EDT need across multiple industries
- Generate any client specific or regulatory EDT’s (including SEDD)
- Create, manage and generate any EDT’s without IT staff involvement or development
- Allows labs to focus on their core competence while EDT’s because a part of standard process flow
- Seamlessly integrates within the lab’s process workflow
- Applicable to any analytical industry
- Delivered via the internet

Request For More Information
100% quality assurance applied to transmitted data and complete traceability back to the source.
Manual entry of data in the lab is the culprit of countless issues causing
- loss of critical time
- errors in results and conclusions
- lack of data traceability and defensibility
- and more…
EISC’s lab data automation systems eliminate the manual entry of lab data, all the while keeping you and your staff in complete control of your lab data process.
- Saves critical time in the lab…hours, days, weeks, and even months
- Minimizes incidents of human errors due to manual data entry
- 100% data traceability and defensibility
- Makes all data electronic for ease of communication across the lab, institute, or industry
- Facilitates replication and duplication of discovery
- Modular and scalable for QA automation/standardization
- Modular and scalable for additional lab data automation features and functionality
- No change to your lab’s current data process or workflow
- Delivered via the internet
- Accelerates science

Request For More Information
100% quality assurance applied to transmitted data and complete traceability back to the source.